Abstract
Background Homoharringtonine (HHT) is a plant cytotoxic alkaloid derived from the trees of the genus Cephalotaxus. HAG (HHT, low-dose cytarabine, and G-CSF), as a priming regimen, is utilized to treat acute myeloid leukemia (AML). Azacitidine (Aza), a DNA hypomethylation agent, has been a "backbone" for new combinational therapies. However, so far, it has not been well determined the efficacy and safety of adding Aza to HAG regimen.
Methods This is a multi-center, single arm, phase 2 clinical trial done in 17 clinical institutions across China between Aug 2019 and Dec 2021 (ClinicalTrials.gov: NCT04248595). Induction therapy consisted of Aza (75mg/m2/d on days 1-7) was given in combination with the HAG regimen (Fig. 1A). Primary endpoints were complete remission (CR) or complete remission with incomplete hematologic recovery (CRi). Secondary endpoints were overall survival (OS), relapse free survival (RFS), and adverse events (AEs). Mutation screening was conducted through next generation sequencing (NGS) by targeting 58 frequently mutated genes.
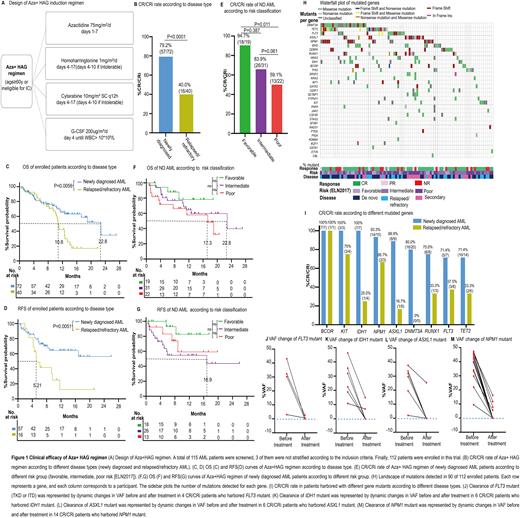
Results A total of 112 patients, including 72 newly diagnosed (ND) (56 de novo, 16 secondary AML [sAML]), and 40 relapsed/refractory (R/R) AML were enrolled. The CR/CRi was achieved in 79.2% (57/72) in the ND and 40.0% (16/40) in R/R AML, respectively (Fig. 1B). CR/CRi were achieved in 80.4% of de novo AML (45/56) and 75.0% of sAML (12/16), respectively. In addition, the median OS and RFS of ND AML patients were 22.8m (95%CI, 12.6 to not reached) and not reached, respectively (Fig. 1C&1D), which were significantly longer than the R/R group (median OS, 10.8m [95%CI, 9.5 to 12.7], P=0.0056; median RFS 5.21m [95%CI, 4.21 to 9.64], P=0.0051) (Fig. 1C&1D). Furthermore, 94.7% (18/19) of patients with favorable-risk reached CR/CRi (Fig. 1E), with median OS and RFS were both not reached (Fig. 1F&1G). 83.9% (26/31) of patients with intermediate-risk reached CR/CRi (Fig. 1E), and the median OS and median RFS were 22.8m (95%CI, 10.5 to not reached) (Fig. 1F) and 16.9m (95%CI, 4.64 to not reached) (Fig. 1G), respectively. Whereas in those with poor-risk, 59.1% (13/22) reached CR/CRi (Fig. 1E), with the median OS 17.3m (95%CI, 3.79 to not reached) (Fig. 1F) and median RFS not reached (Fig. 1G). In this study, infection was the most common non-hematological adverse event, presenting 58.0% (65/112). Common non-hematological AEs of grade 3 or higher includes: infection (38/112, 33.9%), hemorrhage (11/112, 9.82%), fatigue (6/112, 5.36%), hypokalemia (4/112, 3.57%), cardiac arrhythmia (2/112, 1.79%), and fever (2/112, 1.79%). The median duration of neutropenia and thrombocytopenia were 11 d (IQR, 7-19d) and 16d (IQR, 11-25d) among CR/CRi patients. No patients discontinued the induction therapy due to hematological or non-hematological toxicities. The early deaths within 4 weeks of the induction treatment occurred in 1.79% (2/112). Moreover, we detected 228 mutants involving 33 genes in 103 patients at the enrollment. The most frequently mutated genes were DNMT3A (25/103), TET2 (20/103), and NPM1 (18/103) (Fig. 1H), and mostly involved in DNA methylation (DNMT3A, IDH1/2, TET2; 48/103, 46.6%), followed by signal transducers (FLT3, NRAS, KRAS, KIT, PTPN11; 36/103, 35.0%), and transcription regulation (WT1, RUNX1, CEBPA, SETBP1, GATA2; 32/103, 28.2%). Upon Aza+HAG treatment, we observed the high CR/CRi rate in ND patients with mutated BCOR (100%,7/7), KIT (100%,3/3), IDH1 (100%,7/7), NPM1 (93.3%,14/15), ASXL1 (88.9%,8/9), DNMT3A (80. 0%,16/20), RUNX1 (75.0%,6/8), FLT3 (71.4%,5/7), and TET2 (71.4%,10/14) (Fig. 1I). We further observed the clearance of mutated genes after the induction therapy and found the variant allele fraction (VAF) of mutants was dramatically reduced among the CR/CRi patients. Specifically, VAF change of FLT3 mutant from 3 of 4 available patients reduced to <0.01%, with the other one, decreased by 91.9% (Fig. 1J); 4 out of 6 patients with IDH1 mutant reduced to <0.01%, with the other two, decreased 67.6% and 35.9%, respectively (Fig. 1K); 5 out of 6 patients with ASXL1 mutant reduced to <0.01%, with the other one, decreased by 33.4% (Fig. 1L); and 10 out of 14 patients with NPM1 mutant reduced to <0.01%, respectively (Fig. 1M).
Conclusion This study demonstrated that the Aza+HAG regimen is a cost-effective first-line therapy with high efficacy and well tolerance for elderly/unfit AML, especially ND AML patients.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.